Leading the Way in Sarcoma Cancer Treatment
 The Sarcoma Oncology Center in Santa Monica, California, is a renowned cancer treatment and clinical research center led by a team of sarcoma specialists with over 30 years of experience in cancer care. As a premier sarcoma research center, patients have access to some of the newest and most promising experimental medications through our clinical trials.
The Sarcoma Oncology Center in Santa Monica, California, is a renowned cancer treatment and clinical research center led by a team of sarcoma specialists with over 30 years of experience in cancer care. As a premier sarcoma research center, patients have access to some of the newest and most promising experimental medications through our clinical trials.
We are leaders in the field of oncology and among the best for dedicated and innovative treatment solutions for our patients. We partner with specialists from prestigious medical institutions including UCLA Medical Center, USC University Hospital, Cedars-Sinai Medical Center, and St. John’s Health Center. We provide our patients with the most effective, comprehensive treatment plans possible, working closely with each patient’s medical providers including primary care doctors, surgeons, pathologists, and radiologists.
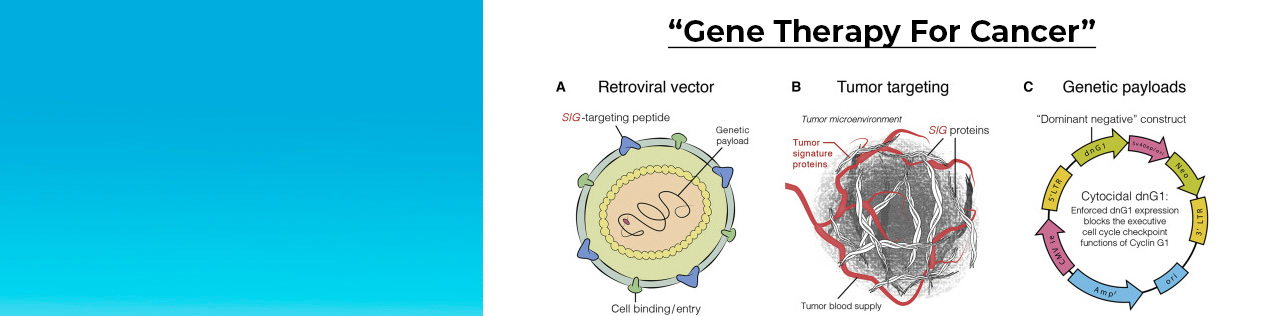
The Sarcoma Oncology Center continuously participates in and leads drug trials for the treatment of all types and stages of sarcoma. Dr. Chawla has conducted groundbreaking research to develop drugs to fight soft tissue sarcomas other cancer, and therapies to ameliorate side effects of standard chemotherapy, all of which have been approved by the FDA.
Please refer to the clinical trials currently taking place at the Sarcoma Oncology Center.
About the Sarcoma Oncology Center
 Founded by Dr. Sant P. Chawla, the Sarcoma Oncology Center is dedicated to providing each and every patient with the highest quality care possible in a warm, compassionate environment. Joining Dr. Chawla are Dr. Doris Quon, Dr. Erlinda Gordon, Dr. Steven Wong, and Ania Moradkhani, who work together to offer the patients of the Sarcoma Oncology Center the best treatments available.
Founded by Dr. Sant P. Chawla, the Sarcoma Oncology Center is dedicated to providing each and every patient with the highest quality care possible in a warm, compassionate environment. Joining Dr. Chawla are Dr. Doris Quon, Dr. Erlinda Gordon, Dr. Steven Wong, and Ania Moradkhani, who work together to offer the patients of the Sarcoma Oncology Center the best treatments available.
Our oncologists are all board certified in internal medicine, hematology, and medical oncology. We also lead and innovate in the fields of hematology and medical oncology and have successfully advanced sarcoma care through our clinical research. We have published or contributed to 500-1000 articles in medical journals, and our physicians are regularly asked to present at national and international conferences, including the annual American Society of Clinical Oncology (ASCO), the annual meeting of Connective Tissue Oncology Society (CTOS) and the biannual meeting of Sarcoma Alliance for Research Through Collaboration (SARC).
Clinical Trials
We are dedicated to defeating cancer through our clinical research in drug development and have become one of the most sought after research centers because of our novel drug therapies for patients. Some of the studies at Sarcoma Oncology have been the first of their kind, such as studies with Aldoxorubicin, Rexin-G, trabectedin, ipilumab and nivolumab. Through our numerous clinical studies, we are constantly striving to provide our patients with better outcomes and cancer-fighting ability.
Contact Our Sarcoma Treatment Specialists Today
If you or a loved one has been diagnosed with a sarcoma, please contact us at 310.552.9999 today to learn more about the Sarcoma Oncology Center and schedule an appointment with one of our leading oncologists. Whether you are seeking a second opinion, interested in learning more about our clinical trials, or just starting your cancer journey, we would like to help you every step of the way.
Learn more about Sarcoma treatment at the Center.
Visiting Medical Student from Brazil

July is Sarcoma Awareness Month
- https://www.curesarcoma.org/sarcoma-awareness-month/
- https://www.aacr.org/patients-caregivers/awareness-months/sarcoma-and-bone-cancer-awareness-month/
- https://natcaf.org/blog/sarcoma-the-forgotten-cancer/
- https://www.cancer.net/cancer-types/sarcomas-soft-tissue/statistics
- https://www.acco.org/sarcoma-awareness-month/
- https://nationaltoday.com/sarcoma-awareness-month/